ISO 27001 Explained: A Guide for Healthtech Startups & Scale-Ups
ISO 27001 for Healthtech: What It Is, Who Needs It, and What Certification Involves
Cordi Mahony•Jan 16, 2026
ISO 27001 Explained: A Guide for Healthtech Startups & Scale-UpsAssuric enables digital health companies and healthcare organisations to exceed compliance requirements, and deploy technology with speed and confidence.

Trusted by both digital health companies and healthcare organisations
We meet you wherever you are with compliance, perform a thorough gap analysis, and upload any existing documentation and evidence
Easily fill any gaps, automate tasks, track compliance, and receive proactive alerts - ensuring requirements are met in record time
Sail through audits, achieve all of the necessary certifications, and enjoy painless maintenance
Navigating digital health compliance is daunting - multiple frameworks, evolving strict regulations, and slow manual processes can stall innovation and drain resources. Here are a few of the biggest challenges...
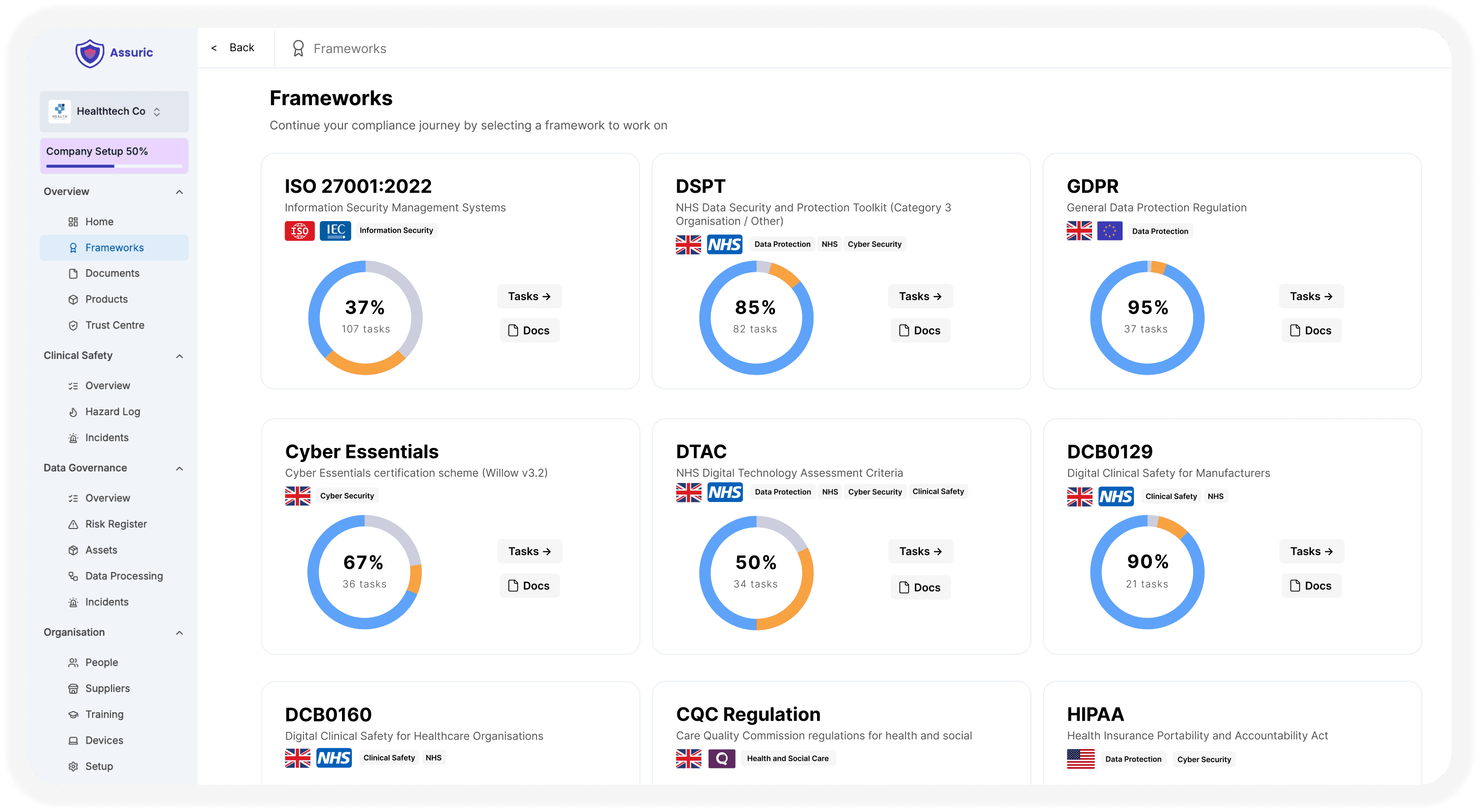
Digital health companies often struggle to meet requirements across GDPR, NHS DTAC, DSPT, DCB0129, ISO standards and more.
Endless spreadsheets, scattered documentation, and repetitive tasks slow you down.
Overlooking clinical hazards or cybersecurity gaps can compromise patient safety and erode trust.
Hiring or training in-house teams or outsourcing everything can be both slow and expensive, especially for SMEs.
Keeping pace with frequent changes in digital health requirements is challenging and creates compliance blind spots.
Without centralised evidence and automated process, preparing for certifications or external reviews is stressful and time-consuming.
We'll help you safeguard patients, gain trust and scale faster
Effortless compliance - from onboarding through to certification and maintenance, so you can redirect resources to where they are needed most
Eliminate painful spreadsheets and manual processes, while avoiding expensive outsourcing or unnecessary hires.
Maintain oversight, implement effective controls, and ultimately reduce the risk of data breaches and patient safety incidents.
Slash timelines from development to certification to deployment - accelerating your path to market and scale.
Designed by clinicians, and clinical safety and data security experts, always with patients and healthcare in mind.
A single source of truth with everything you need for digital health compliance in one place, combined with expert hands-on support at every step.

Information Security Management Systems
The only all-in-one automated...
Safeguard sensitive data through industry-leading compliance, using automated security protocols and continuous monitoring

Ensure consistent quality control across your entire product lifecycle, whilst seamlessly meeting regulatory requirements and passing audits
Identify clinical hazards, implement safety controls, and maintain compliance - protecting patients and reducing risk
Compliance is complex, but our powerful tools simplify it. Discover everything you'll need...
A host of AI features including policy generation, evidence collection, change reporting, gap analyses, and more...
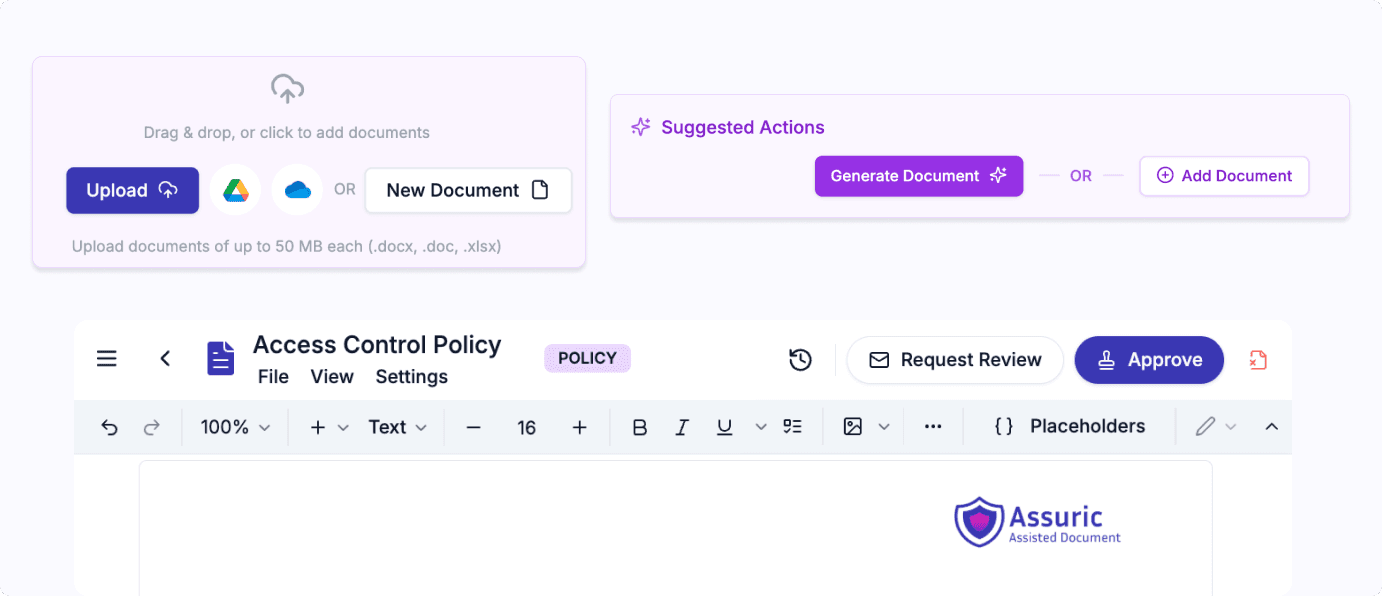
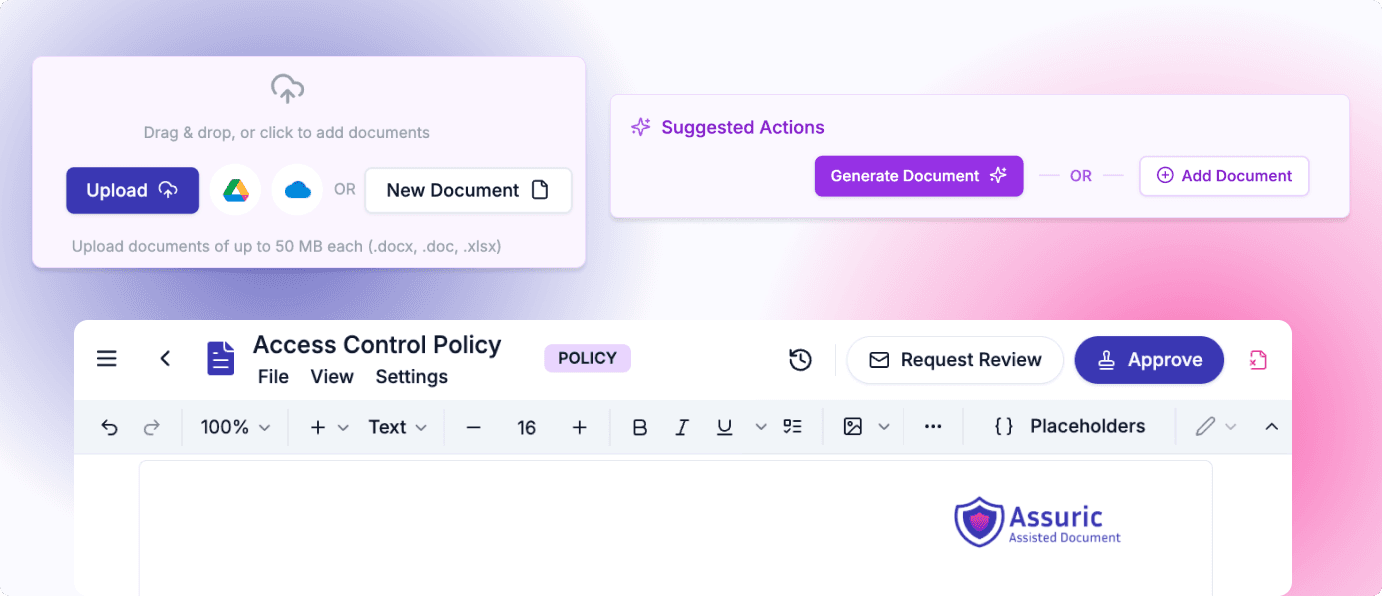
Create, track and update all of your compliance documents automatically. Smart, compliant review and approvals ensuring seamless management
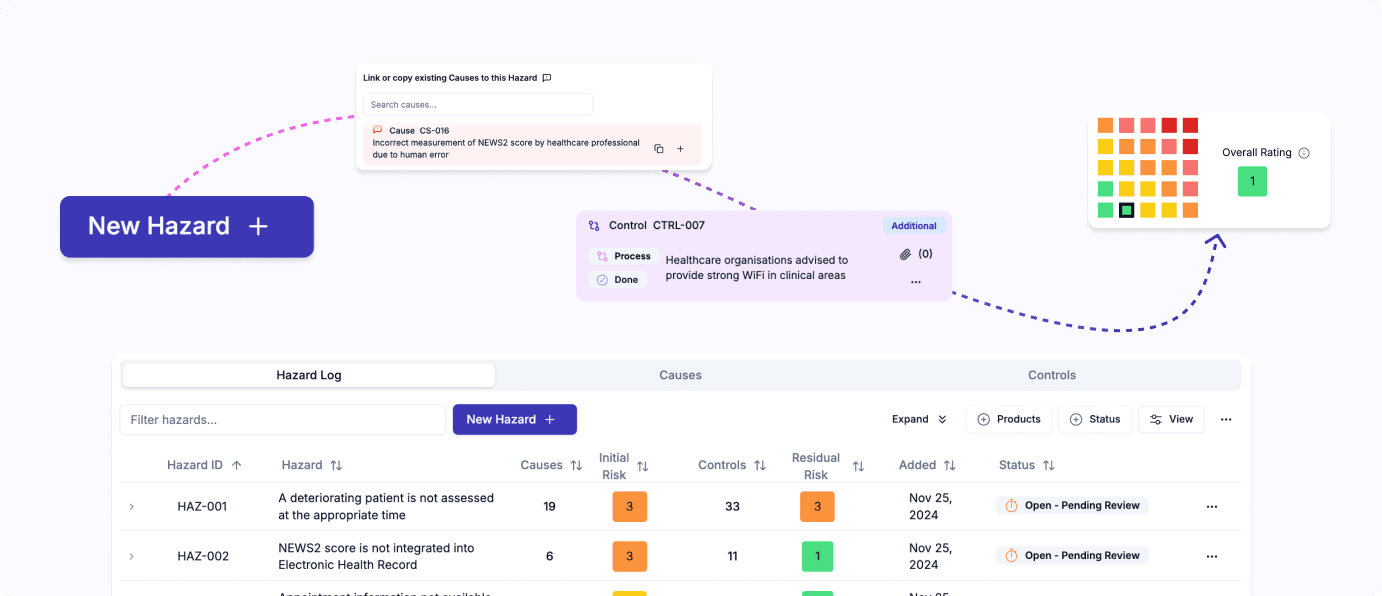
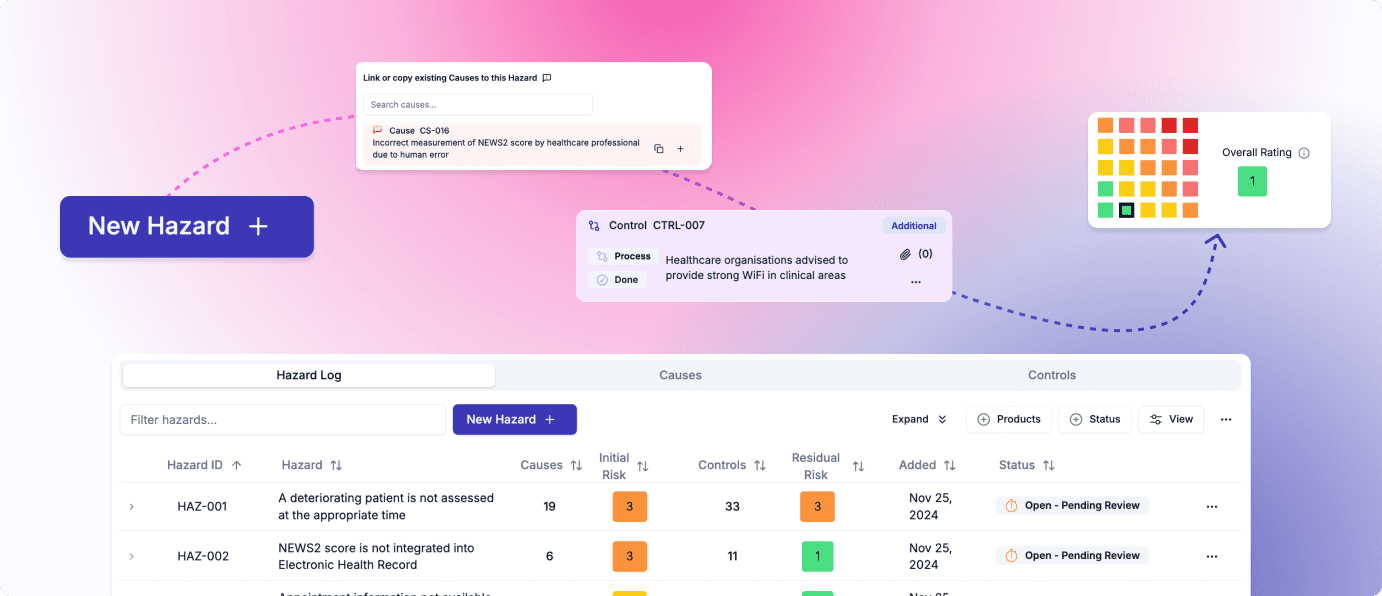
End-to-end clinical safety case management with specialised hazard logs, tracked control implementation, automated report writing, and integrated incident management and post-deployment monitoring
Clinical Safety Overview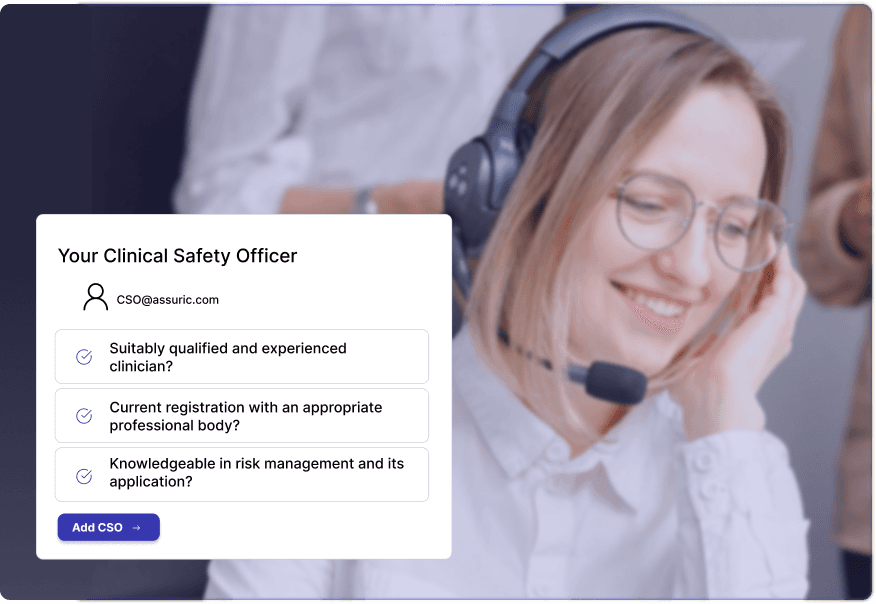
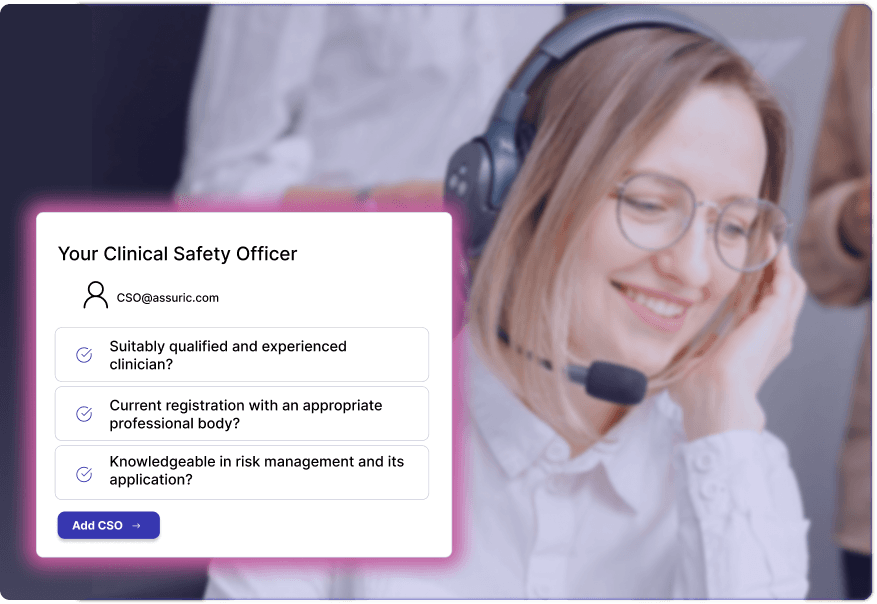
Qualified, experienced Clinical Safety Officers and digital health specialised Data Protection Officers available


Identify and remediate vulnerabilities with CREST-accredited security assessments
Book a test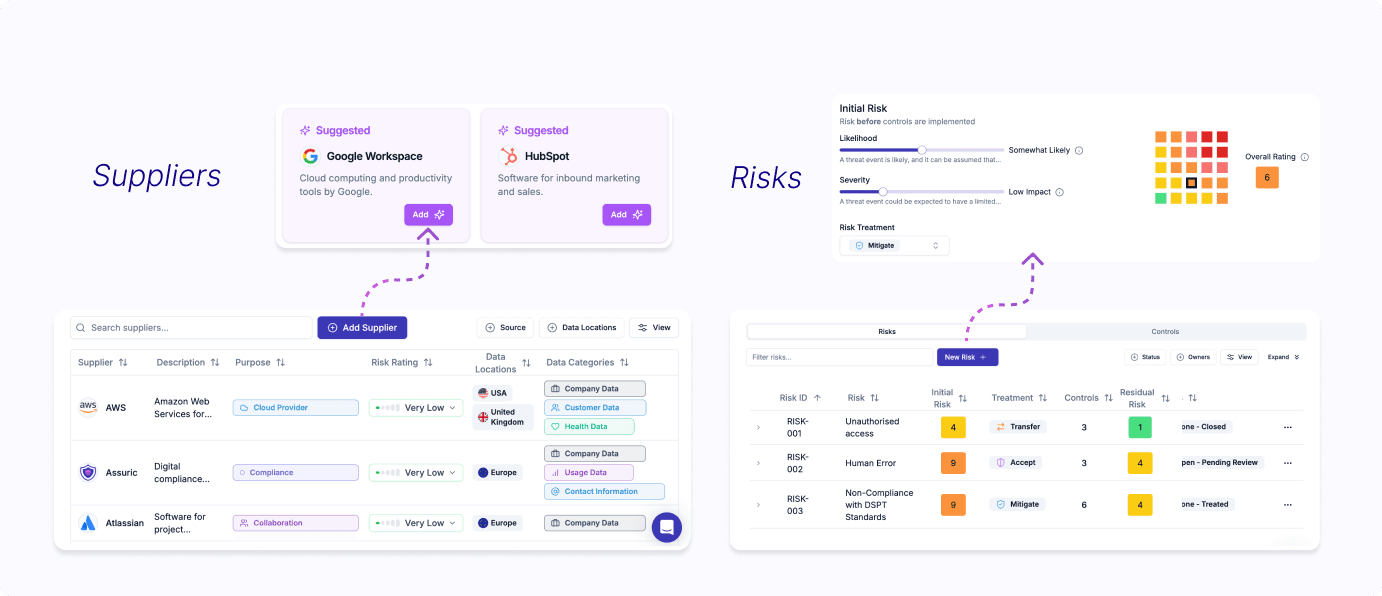
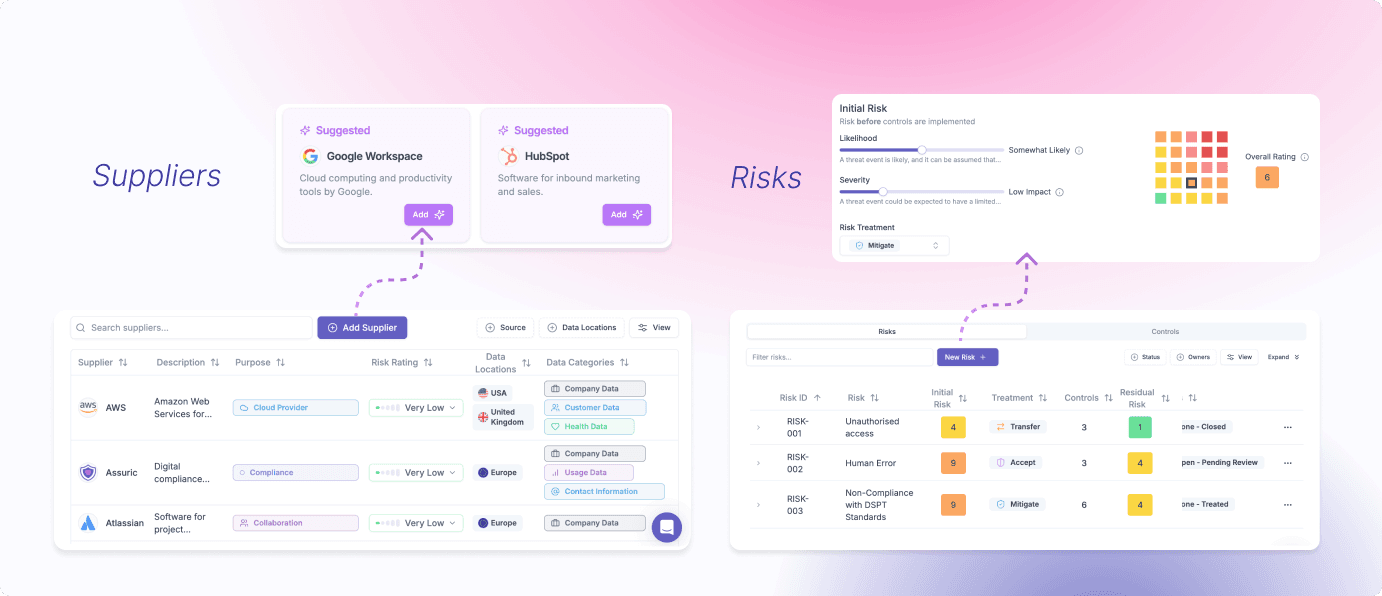
Centralise and automate security control implementation and tracking, asset and supplier management, and specialised security risk management and treatment
Infosec and data protection overview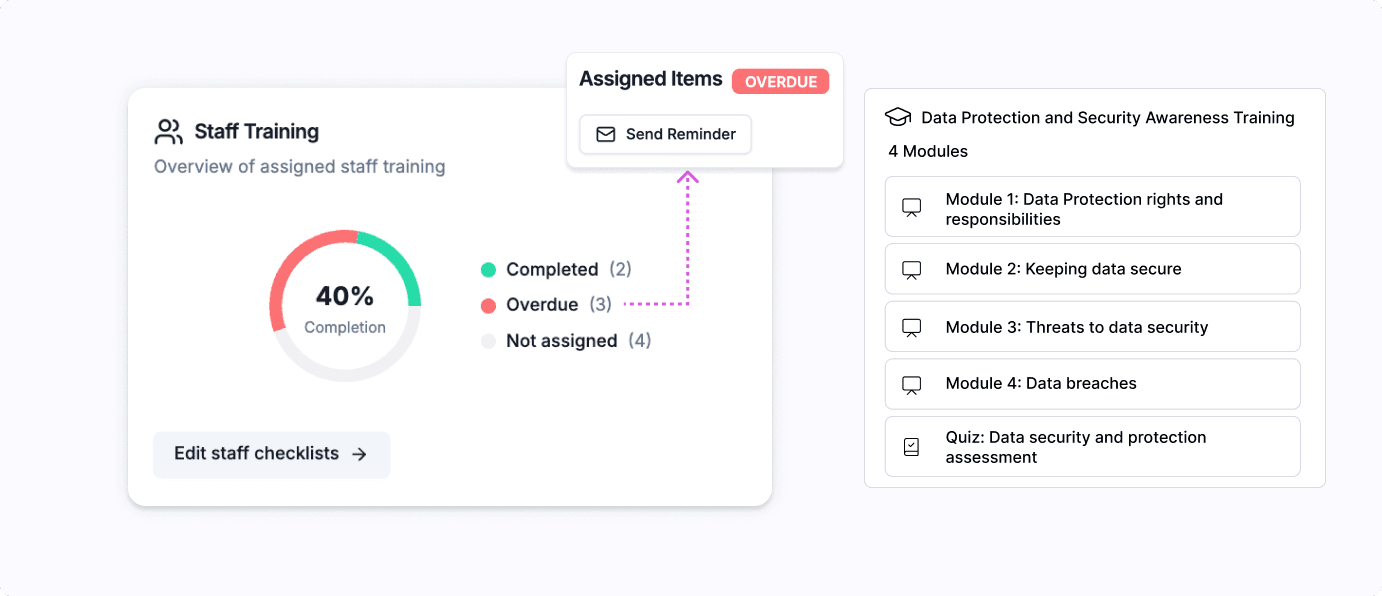
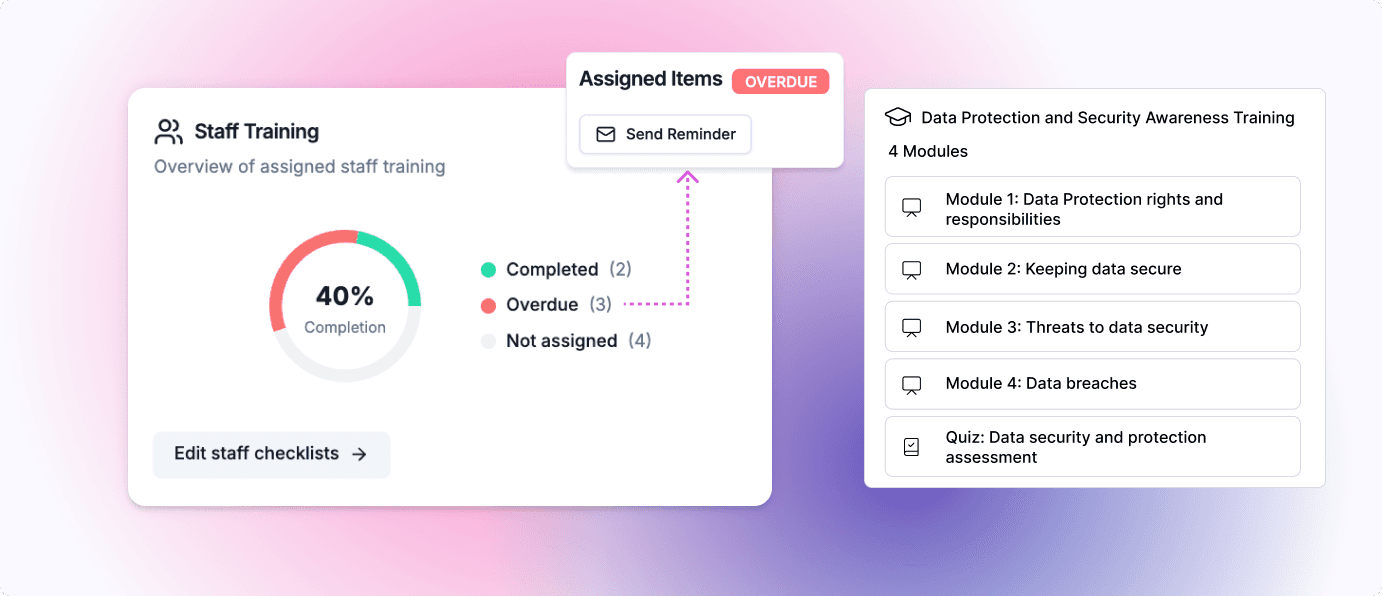
All the necessary staff training you’ll need (both basic and specialist), combined with automated tracking and reminders to ensure compliance
Rapid expert guidance and support as standard, or opt for a fully managed white-glove service with dedicated consultancy
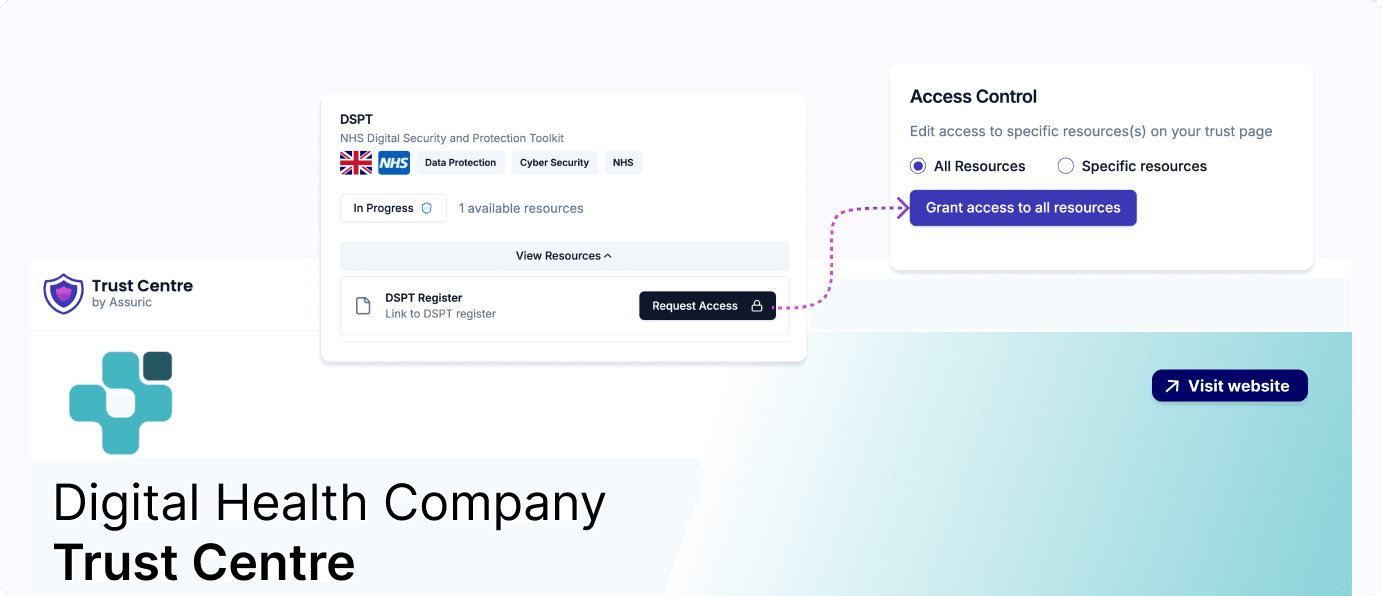
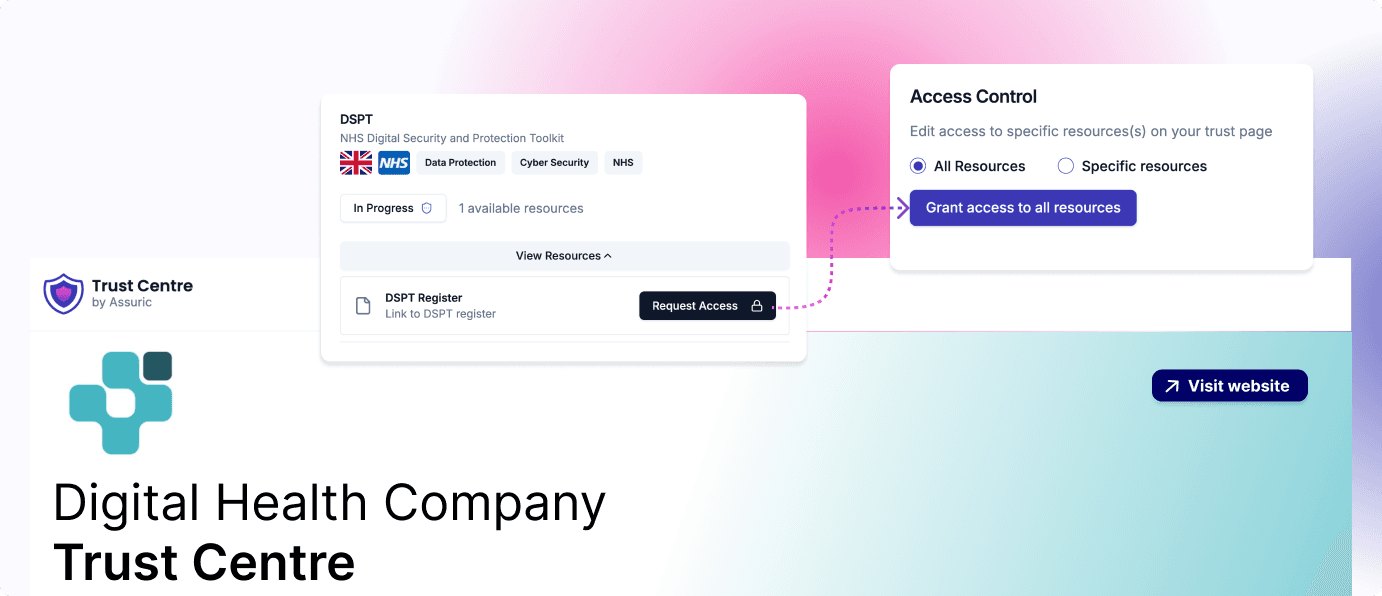
Showcase your compliance position and evidence, build trust with customers and stakeholders, and accelerate your deployment
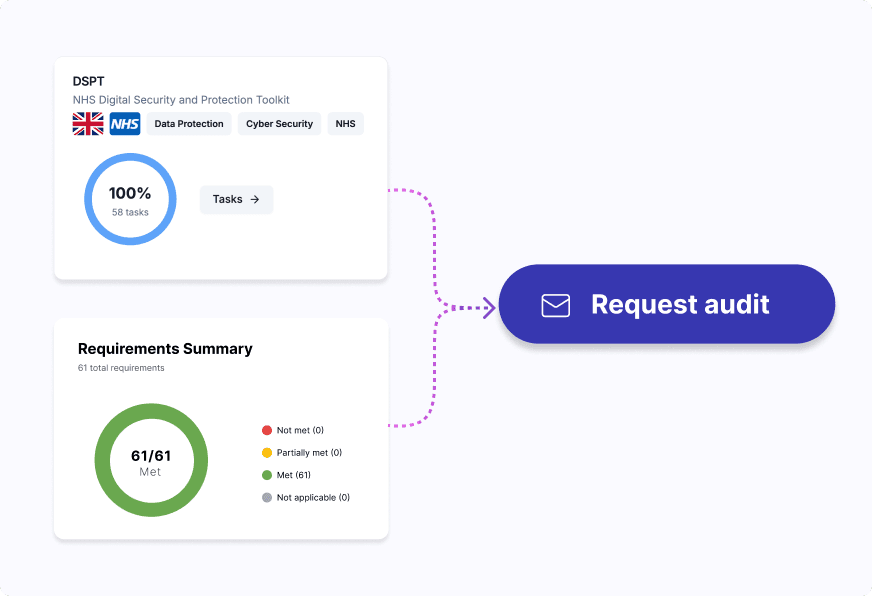
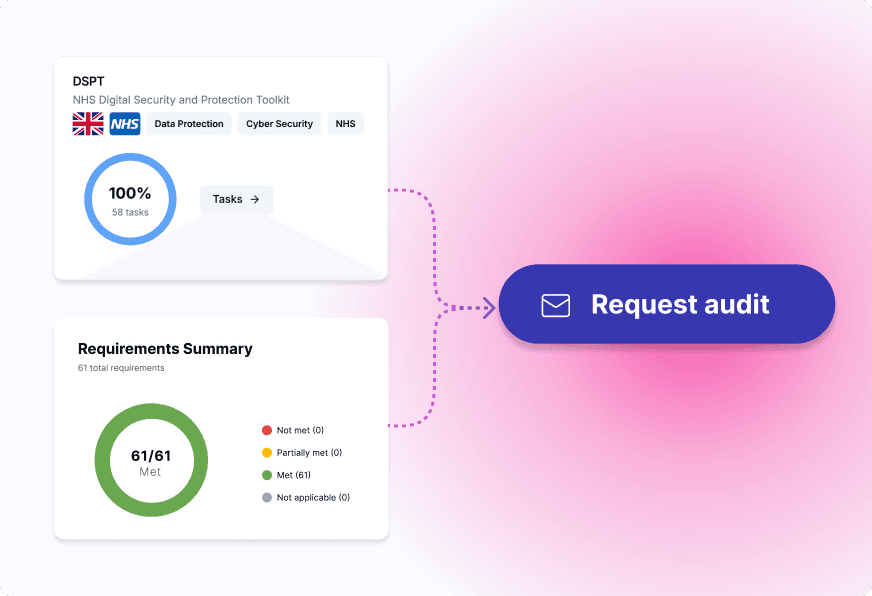
Automate internal audits, streamline external audits and certification processes, and ensure full regulatory compliance
Don’t just take our word for it - discover how we've helped real companies deploy real products into healthcare
Kelly Klifa
CEO at Heim
Assuric has been transformative for Heim as we looked to achieve DCB0129 and DTAC compliance. The platform is easy to use, and the AI tools and automated reminders make previously dreaded compliance tasks a breeze. Paul and Matt supported us every step of the way.
Katie Baker
Director UK & Ireland at Tandem
Assuric has been fantastic in helping us quickly and safely navigate regulatory compliance in the UK. From completing Cybersecurity requirements to DSPT, DCB0129, and DTAC, the team was supportive, extremely knowledgeable, and the platform made everything quick and straightforward. A separate regulatory company we consulted at the beginning even remarked on how quickly we achieved compliance!
Maks Kozarzewski
COO at VitVio
We couldn't speak highly enough of both the Assuric team and the platform itself, which is incredibly easy to use, and with the skill and hardworking nature of the Assuric team. They've been a key component in accelerating our progress and deployments!
Maja Mazur
CEO at Healthnix
Assuric has been such a blessing in getting our DTAC and GDPR compliance done - completing all the documentation and deciding what needs to be done whilst running the business is very hard, but the team really helped us through that. The platform is easy to use, helps keep track of things and it even allows us to coordinate all the team training easily. Highly recommend them!
Dean Mawson
Clinical Director at DPM
Assuric streamlines the process of achieving and maintaining compliance with DCB0129 standards for digital health technologies. The user-friendly interface simplifies collaboration across multidisciplinary teams, while the built-in templates and workflows save significant time and effort during compliance projects. Assuric’s ability to centralise documentation and provide real-time visibility into project progress is particularly beneficial for Clinical Safety Officers and digital project teams, enhancing both efficiency and assurance.
Talk to us to find out how...
Required frameworks spanning data protection, information security, clinical safety and medical device regulation

Application of risk management for medical devices

Quality management system standard for medical devices

Software lifecycle processes for medical device software

Regulatory standards for care quality in UK health and social care services

US-based framework of information security controls by the AICPA

Globally recognised quality management system (QMS) standard

International standard for AI management system (AIMS)
Add your own custom framework or adapt from existing
Whether you're a digital health company or a healthcare organisation, big or small















Seamlessly integrate your existing workflows and tools, to automate everything from security monitoring to incident management.

ISO 27001 for Healthtech: What It Is, Who Needs It, and What Certification Involves
Cordi Mahony•Jan 16, 2026
ISO 27001 Explained: A Guide for Healthtech Startups & Scale-Ups
A practical overview of NHS DTAC, breaking down the requirements, compliance process, and what digital health innovators need to know before selling into the NHS.
Cordi Mahony•Dec 15, 2025
What Is DTAC? A Guide to the NHS Digital Technology Assessment CriteriaGoodbye manual processes, hello automation. Let Assuric manage compliance and security, so you can focus on growth.